Exploring the link between autophagy and cognitive decline offers valuable insights into preserving brain health and preventing neurodegenerative diseases. Autophagy, a cellular recycling process, plays a vital role in maintaining cellular health, while cognitive decline, marked by reduced cognitive abilities, can be a consequence of aging or neurodegenerative conditions.
Contents
Introduction to Autophagy and Cognitive Decline
The intricate relationship between autophagy and cognitive decline has become an increasingly important area of study in recent years. Autophagy, the body’s natural recycling process, plays a critical role in maintaining cellular health by breaking down and recycling damaged cellular components.
On the other hand, cognitive decline, which is characterized by a decrease in cognitive abilities such as memory, attention, and problem-solving skills, can arise from age-related changes or neurodegenerative diseases like Alzheimer’s and Parkinson’s. Understanding the connection between these two phenomena could potentially unlock new strategies for preserving brain health and preventing the onset of cognitive decline.
Definition of Autophagy
Autophagy, derived from the Greek words “auto” (self) and “phagy” (eating), is a highly conserved cellular process that helps maintain cellular homeostasis. It involves the degradation and recycling of cellular components such as proteins, lipids, and organelles in response to various stresses, including nutrient deprivation, hypoxia, and pathogen invasion. Autophagy ensures that cells can adapt to changing conditions and maintain their integrity and function [1].
Definition of Cognitive Decline
Cognitive decline refers to a gradual deterioration of cognitive abilities, including memory, attention, language, and problem-solving skills. It can manifest as a natural part of the aging process, known as age-related cognitive decline, or it can be the result of neurodegenerative diseases like Alzheimer’s, Parkinson’s, or Huntington’s disease. Cognitive decline can significantly impact an individual’s daily functioning, independence, and quality of life.
Importance of Studying the Connection
The connection between autophagy and cognitive decline is a vital area of research due to the potential implications for developing new treatments and preventive measures for neurodegenerative diseases. As the global population continues to age, the prevalence of cognitive decline and related disorders is expected to rise, placing a significant burden on healthcare systems and society.
By understanding how autophagy influences brain function and contributes to cognitive decline, researchers may be able to identify novel therapeutic targets and develop strategies to maintain brain health and prevent cognitive decline.
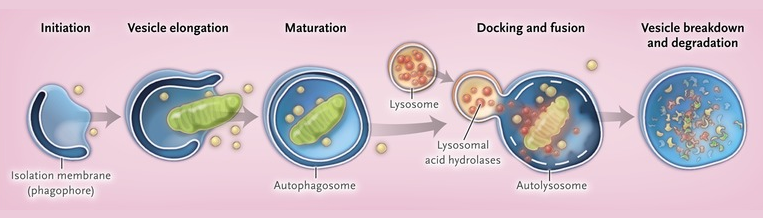
The Process of Autophagy
Autophagy is an essential cellular process that helps maintain homeostasis and ensure the proper functioning of cells. By examining the different aspects of autophagy, we can better understand its role in cellular health and its connection to cognitive decline.
Cellular Recycling Mechanism
Autophagy serves as the cell’s recycling system, responsible for breaking down and recycling damaged or unnecessary cellular components. This process is initiated when a double-membrane vesicle, known as an autophagosome, engulfs the targeted components. The autophagosome then fuses with a lysosome, a cellular organelle containing hydrolytic enzymes that degrade the engulfed material. The resulting products, such as amino acids, fatty acids, and nucleotides, are released back into the cytoplasm for reuse, ensuring that the cell maintains an optimal balance of resources and energy.
Role in Cellular Health
Autophagy plays a critical role in maintaining cellular health by clearing away damaged or dysfunctional cellular components, such as misfolded proteins, damaged organelles, and invading pathogens [2]. By doing so, it prevents the accumulation of harmful substances and ensures the cell’s optimal functioning. Autophagy also helps cells adapt to changing conditions and stressors, such as nutrient deprivation or hypoxia, by providing an alternative source of energy and building blocks for cellular repair and growth.
Activation of Autophagy
Autophagy can be activated through various signaling pathways, many of which are regulated by nutrient availability and cellular stress. One well-known regulator of autophagy is the mammalian target of rapamycin (mTOR) pathway, which inhibits autophagy under nutrient-rich conditions and promotes cell growth and proliferation.
Conversely, when nutrients are scarce or cellular stress is present, the mTOR pathway is inhibited, allowing autophagy to be activated. Other factors, such as cellular energy levels, oxidative stress, and the presence of misfolded proteins, can also influence the activation of autophagy.
Cognitive Decline: Causes and Symptoms
Cognitive decline encompasses a range of conditions that can impact an individual’s cognitive abilities, including memory, attention, language, and problem-solving skills. By understanding the causes and symptoms of cognitive decline, we can better comprehend its connection to autophagy and how these factors may influence brain health.
Age-Related Cognitive Decline
Age-related cognitive decline is a natural part of the aging process, characterized by a gradual decline in cognitive abilities as we grow older. This decline is often subtle and may manifest as forgetfulness, decreased attention span, or slower processing speed. While age-related cognitive decline is not considered a disease, it can still impact an individual’s quality of life and daily functioning.
Neurodegenerative Diseases
Neurodegenerative diseases are a group of disorders characterized by the progressive degeneration of nerve cells in the brain, leading to cognitive decline and functional impairment. Some of the most common neurodegenerative diseases include:
Alzheimer’s Disease
Alzheimer’s disease is the most common cause of dementia, characterized by the buildup of amyloid-beta plaques and tau tangles in the brain. These abnormal protein accumulations disrupt neural communication and lead to the loss of neurons, resulting in memory loss, confusion, and impaired judgment.
Parkinson’s Disease
Parkinson’s disease is a movement disorder primarily affecting the dopamine-producing neurons in a specific area of the brain called the substantia nigra. While Parkinson’s is primarily known for its motor symptoms, such as tremors and rigidity, it can also lead to cognitive decline, including memory problems, difficulty with attention, and slowed thinking.
Huntington’s Disease
Huntington’s disease is a genetic disorder caused by a mutation in the huntingtin gene. This mutation leads to the production of a toxic protein that damages neurons, resulting in motor, cognitive, and psychiatric symptoms. Cognitive decline in Huntington’s disease can manifest as difficulties with attention, memory, planning, and problem-solving.
Symptoms of Cognitive Decline
The symptoms of cognitive decline can vary depending on the underlying cause and the severity of the condition. Some common symptoms associated with cognitive decline include:
- Memory loss, including forgetfulness and difficulty recalling recent events
- Difficulty concentrating and maintaining attention
- Problems with language, such as word-finding difficulties or reduced vocabulary
- Impaired problem-solving and decision-making abilities
- Changes in mood, including increased irritability, anxiety, or depression

How Autophagy Affects Brain Function
The connection between autophagy and cognitive decline lies in the essential role autophagy plays in maintaining brain function. By examining the different ways autophagy affects brain function, we can better understand its role in cognitive decline and identify potential targets for intervention.
Cellular Maintenance in Neurons
Neurons, the primary cells responsible for transmitting information in the brain, are highly reliant on autophagy for their maintenance and survival. As neurons are post-mitotic cells, meaning they do not divide, they must effectively manage and remove damaged components to maintain optimal function. Autophagy ensures the clearance of damaged organelles, misfolded proteins, and other cellular debris, thereby contributing to overall neuronal health and function.
Clearance of Misfolded Proteins
Misfolded proteins can be highly detrimental to neuronal function, as they can form toxic aggregates that interfere with cellular processes and eventually lead to cell death. Autophagy plays a crucial role in clearing misfolded proteins from neurons, preventing the accumulation of these toxic aggregates [3]. Dysfunctional autophagy has been implicated in several neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, where the accumulation of misfolded proteins is a hallmark feature.
Mitochondrial Quality Control
Mitochondria, the energy-producing structures within cells, are essential for neuronal function due to the high energy demands of the brain. Autophagy contributes to mitochondrial quality control by removing damaged mitochondria through a specific process called mitophagy [4]. This process ensures that neurons maintain a healthy population of functional mitochondria, which is critical for neuronal health and the prevention of cognitive decline. Dysfunctional mitophagy has been implicated in various neurodegenerative diseases, highlighting the importance of autophagy in maintaining brain health.
Autophagy and Neurodegenerative Diseases
The role of autophagy in neurodegenerative diseases has become a critical area of study, as impairments in autophagy have been implicated in the pathogenesis of several such conditions. By exploring the role of autophagy in specific neurodegenerative diseases, we can gain insights into potential therapeutic targets and strategies for preventing or mitigating cognitive decline.
Alzheimer’s Disease
Alzheimer’s disease is characterized by the accumulation of amyloid-beta plaques and tau tangles in the brain, both of which contribute to neuronal dysfunction and cognitive decline [5].
Amyloid-β Plaques
Amyloid-beta plaques result from the aggregation of amyloid-beta peptides, which are generated from the amyloid precursor protein through proteolytic cleavage. Autophagy has been shown to play a role in the degradation and clearance of amyloid-beta peptides, preventing their accumulation in the brain. Dysfunctional autophagy in Alzheimer’s disease can lead to increased amyloid-beta production and plaque formation, contributing to disease progression.
Tau Tangles
Tau tangles are aggregates of hyperphosphorylated tau protein, which disrupt the normal functioning of neurons and lead to their degeneration. Autophagy has been implicated in the clearance of hyperphosphorylated tau, suggesting that impaired autophagy may contribute to the accumulation of tau tangles in Alzheimer’s disease.
Autophagy Dysfunction in Alzheimer’s
Several studies have shown that autophagy is impaired in the brains of Alzheimer’s patients, with reduced autophagosome formation and lysosomal dysfunction. This impairment in autophagy contributes to the accumulation of toxic protein aggregates and neuronal degeneration, leading to cognitive decline. Restoring autophagy function has been proposed as a potential therapeutic strategy for Alzheimer’s disease.
Parkinson’s Disease
Parkinson’s disease is characterized by the loss of dopamine-producing neurons in the substantia nigra and the presence of Lewy bodies, which are primarily composed of aggregates of alpha-synuclein protein [6].
Alpha-Synuclein Aggregates
Alpha-synuclein is a protein that plays a role in synaptic function, and its aggregation leads to the formation of toxic Lewy bodies in Parkinson’s disease. Autophagy has been shown to be involved in the clearance of alpha-synuclein aggregates, suggesting that dysfunctional autophagy may contribute to the accumulation of these toxic species and the progression of Parkinson’s disease.
Autophagy Dysfunction in Parkinson’s
Impairments in autophagy have been observed in Parkinson’s disease, with evidence pointing to reduced autophagosome formation and impaired lysosomal function. Enhancing autophagy has been proposed as a potential therapeutic approach to promote the clearance of alpha-synuclein aggregates and mitigate the progression of Parkinson’s disease.
Huntington’s Disease
Huntington’s disease is a genetic disorder caused by a mutation in the huntingtin gene, leading to the production of a toxic protein that damages neurons and results in motor, cognitive, and psychiatric symptoms [7].
Mutant Huntingtin Protein
The mutant huntingtin protein forms toxic aggregates within neurons, disrupting their normal function and leading to neuronal degeneration. Autophagy has been shown to play a role in the clearance of mutant huntingtin aggregates, suggesting that dysfunctional autophagy may contribute to the pathogenesis of Huntington’s disease.
Autophagy Dysfunction in Huntington’s
Studies have found impairments in autophagy in the brains of Huntington’s disease patients, with reduced autophagosome formation and lysosomal dysfunction. Restoring autophagy function has been proposed as a potential therapeutic strategy for Huntington’s disease, promoting the clearance of mutant huntingtin aggregates and slowing disease progression.
Enhancing Autophagy to Prevent Cognitive Decline
Given the essential role of autophagy in maintaining brain health and its connection to neurodegenerative diseases, enhancing autophagy has been proposed as a potential strategy for preventing cognitive decline. Various interventions have been suggested to boost autophagy, which could lead to novel therapeutic approaches for preserving brain function and mitigating the progression of neurodegenerative diseases.
Pharmacological Interventions
Several pharmacological agents have been identified as potential enhancers of autophagy, showing promise in preclinical studies for their ability to boost autophagy and prevent cognitive decline [8].
Rapamycin
Rapamycin is a potent inhibitor of the mTOR pathway, which is a major regulator of autophagy. By inhibiting mTOR, rapamycin can enhance autophagy and promote the clearance of toxic protein aggregates. Preclinical studies have shown that rapamycin treatment can ameliorate cognitive deficits in animal models of Alzheimer’s and Parkinson’s diseases, suggesting its potential as a therapeutic agent for neurodegenerative disorders.
Small Molecule Activators
Several small molecules have been identified as potential autophagy enhancers, including trehalose, spermidine, and resveratrol. These molecules have been shown to activate autophagy through various signaling pathways and have demonstrated neuroprotective effects in preclinical studies.
Lifestyle and Dietary Interventions
Lifestyle and dietary interventions are another promising avenue for enhancing autophagy and promoting brain health.
Caloric Restriction
Caloric restriction, which involves reducing daily caloric intake without causing malnutrition, has been shown to activate autophagy and extend lifespan in various organisms. Studies have also found that caloric restriction can improve cognitive function and reduce the risk of neurodegenerative diseases in animal models, potentially through the activation of autophagy.
Intermittent Fasting
Intermittent fasting is a dietary intervention that alternates between periods of fasting and normal food intake. This dietary strategy has been shown to activate autophagy and improve cognitive function in animal models, suggesting its potential as a preventive measure against cognitive decline.
Exercise
Regular physical exercise has been shown to have numerous health benefits, including the activation of autophagy. Exercise-induced autophagy may contribute to the neuroprotective effects of exercise, such as the reduction of cognitive decline and the risk of neurodegenerative diseases. Incorporating regular exercise into one’s lifestyle could potentially help maintain brain health and prevent cognitive decline.
Future Directions and Challenges
While enhancing autophagy holds promise as a strategy for preventing cognitive decline, several challenges remain to be addressed. These include the development of safe and effective pharmacological agents, determining the optimal dosage and duration of treatment, and understanding the long-term effects of autophagy enhancement on brain health. Further research is needed to fully elucidate the therapeutic potential of autophagy enhancement for cognitive decline and neurodegenerative diseases.
References
[1] Autophagic Mechanisms that Modulate Metabolism
[2] Autophagy in healthy aging and disease
[3] Autophagy and misfolded proteins in neurodegeneration
[4] Molecular Mechanisms of Mitochondrial Autophagy/Mitophagy
[5] Alzheimer’s Disease | Stanford Health Care
[6] Parkinson’s Disease – Symptoms, Diagnosis and Treatment
[7] Huntington’s disease – Symptoms and causes
[8] Pharmacological modulation of autophagy: therapeutic potential

