Recent scientific advancements have uncovered that epigenetics, the study of heritable changes in gene expression without alterations to the underlying DNA sequence, plays a pivotal role in age-related gradual decline in cognitive function.
Contents
- Introduction to Epigenetics and Age-Related Cognitive Decline
- Epigenetic Mechanisms Influencing Cognitive Decline
- Key Epigenetic Targets for Age-Related Cognitive Decline
- Lifestyle Factors Affecting Epigenetic Modifications and Cognitive Decline
- Potential Epigenetic Interventions for Age-Related Cognitive Decline
- References
Introduction to Epigenetics and Age-Related Cognitive Decline
As we age, many of us experience cognitive decline, which can manifest as memory loss, slowed thinking, and reduced learning capacity. Recent research has shown that epigenetics, the study of heritable changes in gene expression without alterations to the DNA sequence itself, plays a significant role in this decline.
Definition of Epigenetics
Epigenetics refers to the study of changes in gene expression that are not caused by alterations in the DNA sequence itself [1]. These changes can be heritable and are influenced by a variety of factors, including environmental exposure, diet, and lifestyle choices. Epigenetic modifications, such as DNA methylation and histone modifications, can alter the way genes are expressed, ultimately affecting various biological processes and functions.
Overview of Age-Related Cognitive Decline
Age-related cognitive decline is a natural process that occurs as we grow older. It involves a gradual decline in cognitive functions such as memory, attention, and problem-solving. While some degree of cognitive decline is considered normal with aging, more severe cases can lead to neurodegenerative diseases like Alzheimer’s and other forms of dementia.
Connection between Epigenetics and Cognitive Decline
Research has shown that epigenetic modifications play a significant role in the development and progression of age-related cognitive decline [2]. These modifications can impact various processes within the brain, including neuroplasticity, inflammation, and the expression of genes related to memory and learning.
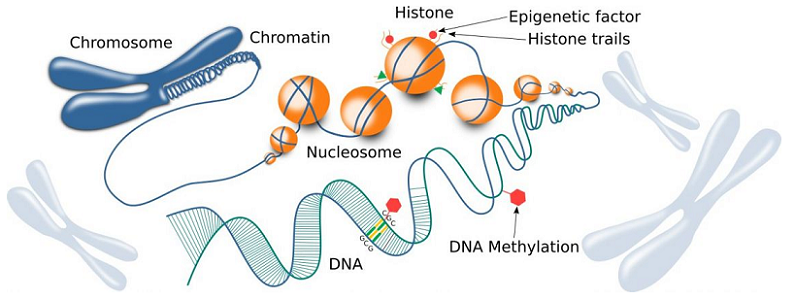
Epigenetic Mechanisms Influencing Cognitive Decline
Various epigenetic mechanisms have been identified as key contributors to age-related cognitive decline, including DNA methylation, histone modifications, and non-coding RNA molecules.
DNA Methylation
DNA methylation is an epigenetic modification that involves the addition of a methyl group to the DNA molecule, usually at a cytosine base. This process can regulate gene expression by affecting the accessibility of DNA to transcription factors, either silencing or activating specific genes [3].
Changes with Aging
As we age, global DNA methylation levels tend to decrease, while specific gene promoters may become hypermethylated. These changes can lead to alterations in gene expression, potentially contributing to age-related cognitive decline.
Impact on Cognitive Function
DNA methylation has been implicated in various aspects of cognitive function, including memory consolidation, synaptic plasticity, and neurogenesis. Altered methylation patterns in specific genes, such as BDNF and CREB, have been linked to cognitive decline in both animal models and human studies.
Histone Modifications
Histone modifications are another important epigenetic mechanism affecting cognitive decline. Histones are proteins that DNA wraps around to form a structure called chromatin. The addition or removal of chemical groups, such as acetyl groups, to histone proteins can change the structure of chromatin and ultimately influence gene expression [4].
Relationship with Cognitive Decline
Histone acetylation has been associated with increased gene expression and is typically considered a marker of active chromatin. Conversely, histone deacetylation is associated with gene silencing. Imbalances in histone acetylation and deacetylation have been linked to age-related cognitive decline, with evidence suggesting that promoting histone acetylation may improve cognitive function in animal models.
Non-Coding RNA Molecules
Non-coding RNA molecules are RNA molecules that do not code for proteins but play important roles in gene regulation. Examples include microRNAs (miRNAs) and long non-coding RNAs (lncRNAs). These molecules can influence gene expression by interacting with DNA, RNA, or proteins [5].
Involvement in Age-Related Cognitive Decline
Non-coding RNA molecules have been implicated in the regulation of genes involved in cognitive function and aging. Dysregulation of specific non-coding RNAs has been associated with age-related cognitive decline, suggesting that they may serve as potential therapeutic targets.
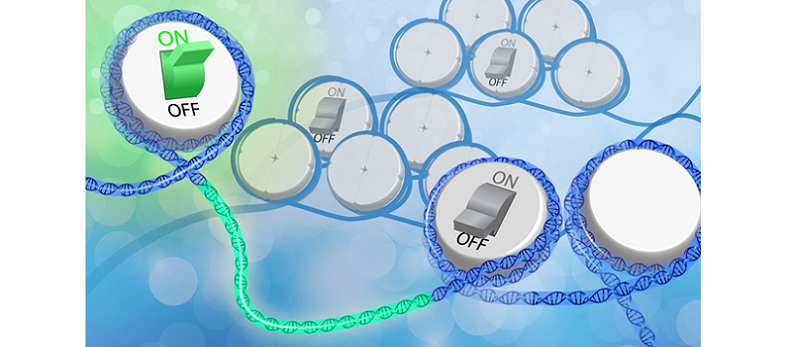
Key Epigenetic Targets for Age-Related Cognitive Decline
Understanding the specific epigenetic targets involved in age-related cognitive decline can help researchers develop targeted interventions. Some of the most important targets include memory-related genes, inflammatory genes, and those involved in neurogenesis and synaptic plasticity.
Memory-Related Genes
Brain-derived neurotrophic factor (BDNF) is a crucial protein involved in the growth, survival, and differentiation of neurons. It also plays a key role in synaptic plasticity, learning, and memory. Epigenetic regulation of the BDNF gene, particularly through DNA methylation and histone modifications, has been linked to age-related cognitive decline. Studies have shown that increasing BDNF levels through epigenetic modifications can improve cognitive function in animal models [6].
CREB and Its Epigenetic Control
The cAMP response element-binding protein (CREB) is another critical gene involved in memory formation and synaptic plasticity. Epigenetic regulation of CREB, such as through DNA methylation, can influence cognitive function. Research has demonstrated that alterations in CREB methylation are associated with age-related cognitive decline and neurodegenerative diseases.
Inflammatory Genes
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) is a protein complex involved in the regulation of inflammation and immune response. Dysregulation of NF-kB has been implicated in the development of age-related cognitive decline and neurodegenerative diseases. Epigenetic modulation of NF-kB, particularly through histone modifications, can influence the expression of inflammatory genes and contribute to cognitive decline [7].
Cytokines and Inflammation in Cognitive Decline
Cytokines are small proteins involved in cell signaling and immune response. Chronic inflammation, marked by elevated levels of pro-inflammatory cytokines, has been linked to age-related cognitive decline. Epigenetic modifications, such as DNA methylation, can regulate the expression of cytokine genes, thereby influencing inflammation and cognitive function.
Epigenetic Regulation of Neurogenesis
Neurogenesis, the process of generating new neurons, is essential for maintaining cognitive function throughout life. Epigenetic mechanisms, including DNA methylation and histone modifications, play a critical role in regulating neurogenesis, particularly in the hippocampus, a brain region crucial for learning and memory.
Synaptic Plasticity and Epigenetic Modifications
Synaptic plasticity, the ability of synapses to strengthen or weaken over time, is crucial for learning and memory. Epigenetic modifications can influence synaptic plasticity by affecting the expression of genes involved in this process. Altered epigenetic regulation of these genes has been implicated in age-related cognitive decline.
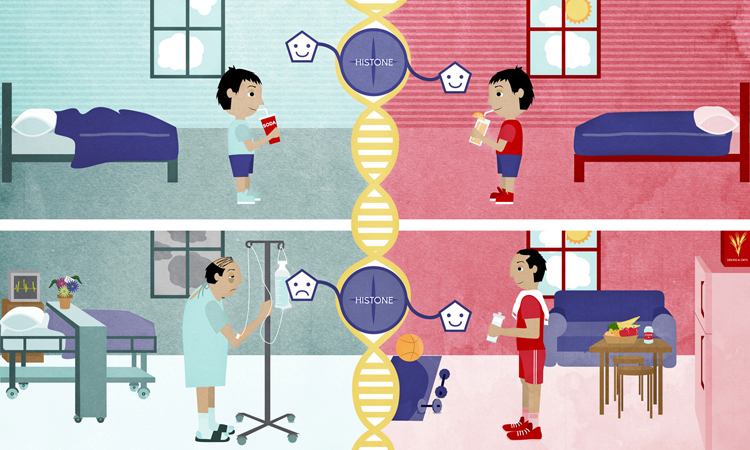
Lifestyle Factors Affecting Epigenetic Modifications and Cognitive Decline
Various lifestyle factors can influence epigenetic modifications and, consequently, age-related cognitive decline. These factors include diet and nutrition, physical activity, and sleep and circadian rhythms.
Diet and Nutrition
Dietary patterns can influence epigenetic modifications and cognitive function [8]. For example, diets high in fruits, vegetables, whole grains, and fish, such as the Mediterranean diet, have been associated with a reduced risk of cognitive decline and dementia. These diets are rich in nutrients that can modulate epigenetic marks and promote brain health.
Specific Nutrients and Their Epigenetic Effects
Certain nutrients have been shown to directly affect epigenetic modifications. For example, folate, vitamin B12, and other B vitamins are involved in the metabolism of homocysteine, a compound that can influence DNA methylation. Omega-3 fatty acids, found in fish and some plant sources, can also modulate epigenetic marks, promoting brain health and cognitive function.
Physical Activity
Regular physical activity has been consistently linked to better cognitive function and a reduced risk of cognitive decline. Exercise can induce epigenetic modifications, such as changes in DNA methylation and histone acetylation, which can influence the expression of genes related to learning, memory, and neuroplasticity.
Exercise-Induced Cognitive Benefits
Exercise has been shown to promote the release of BDNF, increase neurogenesis, and improve synaptic plasticity, which may, in part, be mediated by epigenetic mechanisms. Incorporating regular physical activity into one’s lifestyle can have a positive impact on brain health and cognitive function.
Sleep and Circadian Rhythms
Sleep is essential for maintaining cognitive function, and chronic sleep deprivation has been linked to cognitive decline. Sleep deprivation can lead to epigenetic changes, including alterations in DNA methylation and histone modifications, which can affect the expression of genes related to cognitive function, inflammation, and metabolic processes [9].
Sleep and Cognitive Decline
Maintaining healthy sleep patterns and addressing sleep disorders can help prevent age-related cognitive decline. Ensuring adequate sleep duration and quality may counteract the negative epigenetic effects of sleep deprivation on cognitive function.
Potential Epigenetic Interventions for Age-Related Cognitive Decline
DNA methyltransferase inhibitors are a class of drugs that can prevent or reverse DNA methylation, potentially restoring the expression of genes involved in cognitive function. These drugs are being investigated for their potential to treat age-related cognitive decline and neurodegenerative diseases.
Histone deacetylase inhibitors can promote histone acetylation and, consequently, gene expression. These drugs have shown promise in preclinical studies for their ability to improve cognitive function and are being explored as potential treatments for age-related cognitive decline and neurodegenerative diseases.
Nutraceuticals and Supplements
Curcumin, a compound found in turmeric, has been shown to modulate epigenetic marks, including DNA methylation and histone modifications. It has demonstrated potential for improving cognitive function and reducing inflammation in preclinical studies.
Resveratrol and Its Epigenetic Influence
Resveratrol, a polyphenol found in grapes and red wine, has also been shown to modulate epigenetic marks and promote cognitive function in animal studies. Further research is needed to understand its potential benefits for human cognitive health.
Environmental and Lifestyle Modifications
Chronic stress can lead to detrimental epigenetic changes that contribute to cognitive decline. Incorporating stress reduction techniques, such as mindfulness meditation, yoga, or tai chi, can help counteract these negative effects and promote cognitive health.
Cognitive Training and Brain Stimulation
Cognitive training and non-invasive brain stimulation techniques, such as transcranial direct current stimulation (tDCS), have shown promise in improving cognitive function by modulating neuroplasticity and synaptic plasticity. These interventions may also influence epigenetic marks related to cognitive function.
References
[1] What is Epigenetics? | CDC
[2] Epigenetic Mechanisms in Memory and Cognitive Decline
[3] DNA Methylation and Its Basic Function
[4] An epigenetic hypothesis of aging-related cognitive dysfunction
[5] Epigenetic regulation of aging: implications for interventions of aging and diseases
[6] The Connection Between Autophagy and Cognitive Decline
[7] Aging in the Brain: New Roles of Epigenetics in Cognitive Decline
[8] Epigenetic mechanisms in the development of memory and their involvement in certain neurological diseases
[9] Recent Findings in Alzheimer Disease and Nutrition Focusing on Epigenetics

