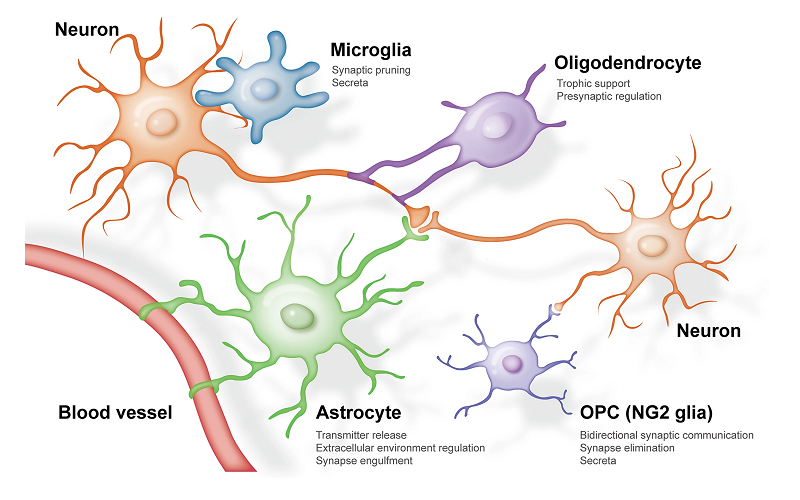
For many years, the intricate dance of neuronal communication was believed to revolve solely around synapses – those crucial junctions where neurons converse. As fascinating as synapses are, recent scientific revelations suggest that they aren’t the only stars in the grand performance of our brain’s communication system. Enter the astrocytes: the often overlooked supporting actors that are now emerging as central players in this intricate ballet. Once relegated to the role of mere “brain glue,” these star-shaped cells are revealing secrets that are reshaping our understanding of cognition, memory, and various neurological disorders.
Contents
- Introduction to Astrocytes and Neuronal Communication
- Understanding the Basics of Astrocytes
- Astrocytes and the Synaptic Environment
- Mechanisms of Astrocyte-Mediated Neuronal Communication
- Astrocytes in Brain Health and Disease
- References
Introduction to Astrocytes and Neuronal Communication
The brain, often hailed as the most complex organ in the human body, relies on billions of neural networks to function. Each thought, sensation, and movement emerges from intricate patterns of communication between these neurons. Historically, the weight of our understanding was placed upon synapses—the connectors that allow neurons to “talk” to each other. Yet, as with any great mystery, the more layers we peel back, the more complexities we uncover. Among these layers, the unsung heroes of our neural networks: the astrocytes.
Definition of Neuronal Communication
Neuronal communication is the process by which neurons exchange information to regulate bodily functions, process sensory information, and execute motor tasks. At its core, this communication happens via electrochemical signals. Neurons send these signals to each other through intricate networks, primarily at specialized junctions known as synapses. It’s a marvel of nature, akin to the world’s most sophisticated electric circuitry, but infinitely more nuanced.
Traditional Understanding of Synapses
Synapses have long been the focal point of neuroscience. They’re the meeting points where neurons exchange information. Think of them as crowded marketplaces, buzzing with activity. In these markets, one neuron releases neurotransmitters—chemical messengers—into a synaptic cleft, which the neighboring neuron then detects. This detection prompts a reaction, allowing the signal to travel onward. Historically, our comprehension of the brain’s functionality was based on these synaptic interactions. Yet, synapses don’t function in isolation. Surrounding them, often overshadowed, are the astrocytes.
Introduction to Astrocytes and Their Previously Underestimated Importance
Astrocytes, deriving their name from the Greek word for “star” due to their star-shaped structure, were traditionally viewed as passive supporters in the neural ensemble. Their role was believed to be maintaining the environment in which neurons functioned, not unlike stagehands in a theatrical production. But emerging research paints a different picture. Rather than silent supporters, astrocytes are dynamic contributors to the communication process, interacting with neurons in ways we’re only beginning to understand [1].
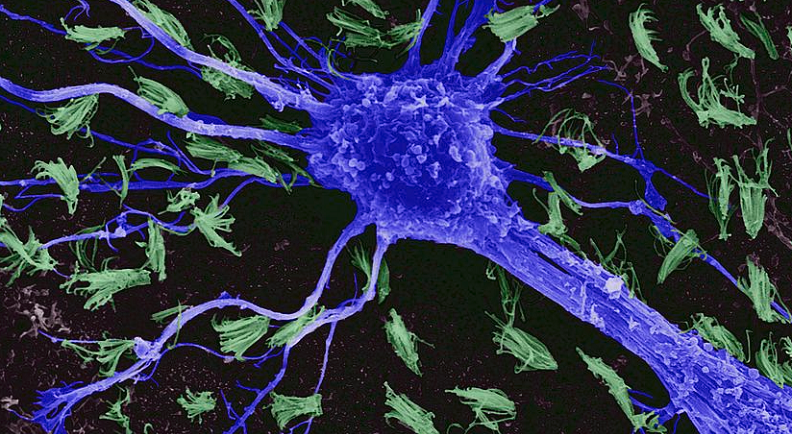
Understanding the Basics of Astrocytes
As we tread the corridors of cognition and delve deeper into the brain’s orchestra, it becomes clear that neurons are not the only musicians. Astrocytes, long considered mere spectators, play a more active and essential role than previously believed. To truly grasp the implications of their functions, it’s crucial to first understand their basic attributes.
Definition and Characteristics of Astrocytes
Astrocytes belong to a family of cells known as glial cells or simply glia. Unlike neurons, which are primarily involved in transmitting information, astrocytes are part of the supportive infrastructure of the nervous system. Their star-shaped structures stretch out in all directions, touching neurons, blood vessels, and other astrocytes. This wide reach allows them to perform various roles, from providing nutrients to neurons to maintaining the blood-brain barrier—a protective boundary that keeps potentially harmful substances from entering the brain.
Distinction Between Astrocytes and Neurons
To appreciate the distinctiveness of astrocytes, one must juxtapose them with neurons. Neurons, with their long axons and dendrites, are designed to transmit electric signals over distances. Their architecture facilitates rapid communication across vast neural networks. Astrocytes, on the other hand, do not generate electrical impulses like neurons. Instead, they use intracellular calcium waves to communicate and influence their environment. Their lack of axons and dendrites, combined with their extensive branching processes, enable them to envelop and interact with multiple synapses simultaneously, positioning them as modulators of synaptic activity.
Evolutionary Significance
Astrocytes are not merely an evolutionary afterthought. In fact, as one moves up the evolutionary ladder, the number and complexity of astrocytes seem to increase, especially in the human brain. This suggests that as brains evolved to handle more complex tasks and store more information, the role of astrocytes became increasingly paramount. Their influence on synaptic function, neuroplasticity (the brain’s ability to reorganize itself), and overall brain health might be among the factors that have given higher mammals, including humans, a cognitive edge [2].
Astrocytes and the Synaptic Environment
Having journeyed through the basics of astrocytes, it’s time to plunge into the heart of the matter: the dynamic relationship between astrocytes and the synapses they envelop. This relationship sheds light on how, contrary to conventional wisdom, astrocytes aren’t just passive observers but active participants in the synaptic symphony of the brain.
Astrocyte’s Proximity to Synapses
Astrocytes, with their intricate and sprawling tendrils, are perfectly positioned to detect and influence neuronal activity. Their processes, delicate and web-like, snuggle close to synapses. This intimate proximity allows them to “eavesdrop” on synaptic conversations, sensing when a neuron fires and subsequently, altering the environment in response. This closeness, coupled with their extensive reach, means a single astrocyte can interact with thousands of synapses simultaneously.
The Tripartite Synapse Concept
To understand the full breadth of astrocyte influence, we must redefine our traditional understanding of synapses. Historically, the synapse was viewed as a two-part structure—the presynaptic neuron, which sends the message, and the postsynaptic neuron, which receives it. This binary model has been expanded upon with the introduction of the tripartite synapse concept.
Traditional Neuronal Synapse (Presynaptic and Postsynaptic Components)
Here, communication occurs when the presynaptic neuron releases neurotransmitters that travel across a gap (synaptic cleft) to bind with receptors on the postsynaptic neuron. This binding elicits an electrical response, which can either excite or inhibit the postsynaptic neuron.
Astrocyte’s Perisynaptic Processes
Enveloping the traditional synapse are astrocyte processes, known as perisynaptic processes. These processes don’t merely serve as insulation; they actively modulate synaptic activity. By releasing and absorbing various chemicals, they can influence the strength and duration of synaptic signals.
Dynamic Interactions Among All Three Components
The tripartite synapse concept underscores a dynamic feedback loop involving all three components. For instance, an astrocyte can detect neurotransmitter release from the presynaptic neuron, respond by releasing its own chemicals (gliotransmitters), and then modulate the activity of both the presynaptic and postsynaptic neurons.
Introduction to the Expansive Role of Astrocytes in Synaptic Function
The more we delve into the world of astrocytes, the more we realize their central role in the synaptic environment. They can amplify, attenuate, or even alter synaptic signals, making them essential modulators of neuronal communication. Their influence stretches from maintaining the balance of ions in the synaptic cleft to controlling the availability of neurotransmitters, ensuring synapses function optimally [3].

Mechanisms of Astrocyte-Mediated Neuronal Communication
Armed with a richer understanding of the interplay between astrocytes and synapses, let’s delve deeper into the mechanics of how astrocytes modulate neural communication. By transcending the traditional boundaries of neural interactions, astrocytes reveal themselves as indispensable custodians and orchestrators of synaptic harmony.
Calcium Signaling in Astrocytes
While neurons use electrical impulses to communicate, astrocytes employ a different mechanism. Their language is based on calcium, a versatile and vital messenger in cellular processes.
Mechanisms of Calcium Wave Propagation
When stimulated—whether by nearby neuronal activity, changes in blood flow, or other astrocytes—astrocytes experience a rise in their intracellular calcium levels. This calcium increase spreads through the astrocyte in a wave-like fashion. Intriguingly, these waves can also propagate to neighboring astrocytes via gap junctions, specialized connections that allow direct cellular communication. This creates a cascading effect, enabling distant astrocytes to “sense” local neural activity.
Impacts on Neuronal Activity
Calcium waves aren’t merely for astrocyte-astrocyte communication; they also play a pivotal role in interacting with neurons. When an astrocyte’s calcium levels surge, it can release gliotransmitters—chemicals that can either excite or inhibit nearby neurons. This adds an additional layer of modulation to the traditional synaptic transmission, fine-tuning the neural message.
Release of Gliotransmitters
Gliotransmitters, as alluded to earlier, are the agents of influence for astrocytes. Their release reshapes the synaptic landscape, providing another dimension to neural communication.
Definition and Examples of Gliotransmitters
Gliotransmitters are chemicals released by astrocytes in response to increased intracellular calcium. Examples include glutamate, ATP, and D-serine. Each of these has distinct effects on nearby neurons, influencing their excitability and response patterns.
Modulation of Synaptic Activity
When released, gliotransmitters can bind to receptors on both presynaptic and postsynaptic neurons. For instance, an astrocyte-derived glutamate might bind to a presynaptic receptor, boosting the release of neurotransmitters and amplifying the message. Conversely, it could also bind to postsynaptic receptors, altering the receiving neuron’s response. This capacity to influence both sides of the synapse underscores the astrocyte’s role as a synaptic maestro, fine-tuning the dialogue between neurons [4].
Uptake and Recycling of Neurotransmitters
Beyond releasing chemicals, astrocytes are also adept at cleanup operations, ensuring the synaptic environment remains conducive for communication.
Astrocytes in Glutamate Recycling
Glutamate, a primary excitatory neurotransmitter, must be rapidly cleared from the synaptic cleft to prevent excessive neuronal stimulation. Enter astrocytes, which swiftly absorb this glutamate. Once inside, it undergoes a transformation into glutamine, which is then released and taken up by neurons to be converted back into glutamate. This recycling process is crucial for sustaining ongoing neural communication.
Impact on Synaptic Efficacy
By controlling the duration neurotransmitters remain in the synaptic cleft, astrocytes influence how effectively neurons can communicate. Too much lingering neurotransmitter can lead to excessive stimulation, while too little can dampen the message. Astrocytes ensure the balance is just right, optimizing synaptic efficacy.
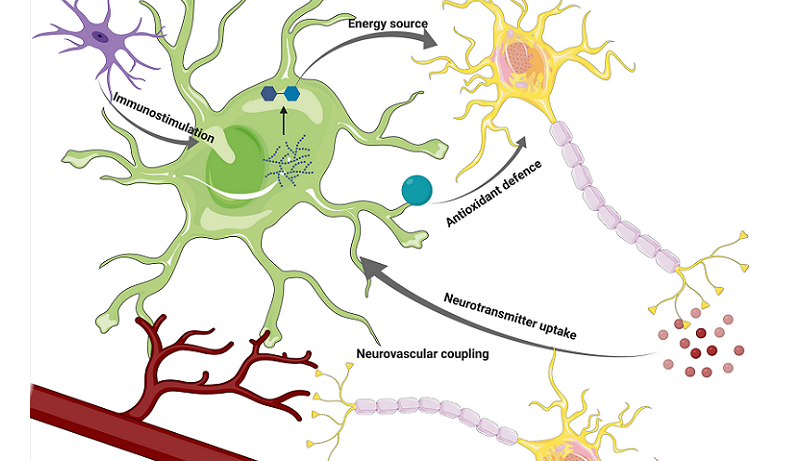
Astrocytes in Brain Health and Disease
As our expedition through the realm of astrocytes continues, we move beyond the microscopic symphonies of individual synapses to explore the macroscopic tapestry of the entire brain. Indeed, the role of astrocytes isn’t limited to facilitating neuron-to-neuron conversations. They play a pivotal role in preserving overall brain health and can significantly influence the progression of various neurological diseases.
Neuroprotection and Brain Repair
Astrocytes are the brain’s unsung custodians. Their protective and regenerative roles underscore their importance in maintaining a healthy neural environment.
Blood-Brain Barrier Maintenance
One of the critical protective mechanisms of the brain is the blood-brain barrier (BBB), a selective barrier that prevents potentially harmful substances in the blood from entering the brain. Astrocytes are pivotal in maintaining and repairing the BBB, ensuring that only specific molecules gain entry while potentially harmful entities are kept at bay [5].
Response to Injury
In the event of brain injury, astrocytes spring into action. They proliferate and migrate to the injury site, forming a protective barrier that prevents the spread of damaging agents. This reactive state, often termed “astrogliosis,” also helps in remodeling the neural environment, paving the way for potential recovery and regeneration.
Neurotrophic Support
Astrocytes are a source of neurotrophic factors—molecules that support neuron survival, growth, and differentiation. By releasing these nurturing compounds, astrocytes help maintain the health and vitality of the surrounding neural tissue.
Astrocytes in Neurological Diseases
While astrocytes are instrumental in sustaining brain health, their dysfunction can also contribute to, or exacerbate, several neurological conditions.
Alzheimer’s Disease
Emerging research hints at the involvement of astrocytes in Alzheimer’s progression. In the disease’s milieu, astrocytes may contribute to inflammation and fail to effectively clear amyloid-beta, a protein fragment that accumulates and forms plaques in Alzheimer’s brains.
Epilepsy
In epileptic conditions, where abnormal electrical activity runs rampant, astrocytes’ roles in modulating synaptic activity and maintaining ionic balance come to the forefront. Dysfunctional astrocytes might contribute to the destabilization of neural networks, facilitating or exacerbating seizure episodes.
Amyotrophic Lateral Sclerosis (ALS)
ALS, a neurodegenerative disorder affecting motor neurons, also witnesses astrocyte involvement. Mutations causing abnormal astrocyte function have been linked to increased motor neuron death, accelerating the disease’s progression.
Potential Therapeutic Implications
Given their profound influence on brain health and disease, astrocytes present tantalizing therapeutic targets.
Astrocyte Modulation for Disease Treatment
By understanding and harnessing the mechanisms by which astrocytes influence neural communication, it might be possible to develop treatments that either correct astrocytic dysfunctions or leverage their protective roles.
Astrocyte Transplantation
Recent strides in stem cell research raise the possibility of generating astrocytes for transplantation. Introducing healthy astrocytes into disease-afflicted regions could offer a novel approach to combatting various neurological disorders.
Drug Targeting to Astrocytes
Recognizing astrocytes’ importance opens up avenues for drug development that specifically target these cells, either to bolster their protective functions or mitigate their deleterious effects.
References
[1] Astrocyte–Neuron Networks: A Multilane Highway of Signaling for Homeostatic Brain Function
[2] The role of astrocyte-mediated plasticity in neural circuit development and function
[3] Dysregulation of Astrocyte–Neuronal Communication in Alzheimer’s Disease
[4] New Form of Astrocyte–Neuron Communication Is Identified
[5] Advances in understanding new roles for astrocytes in the modulation of neuronal activity